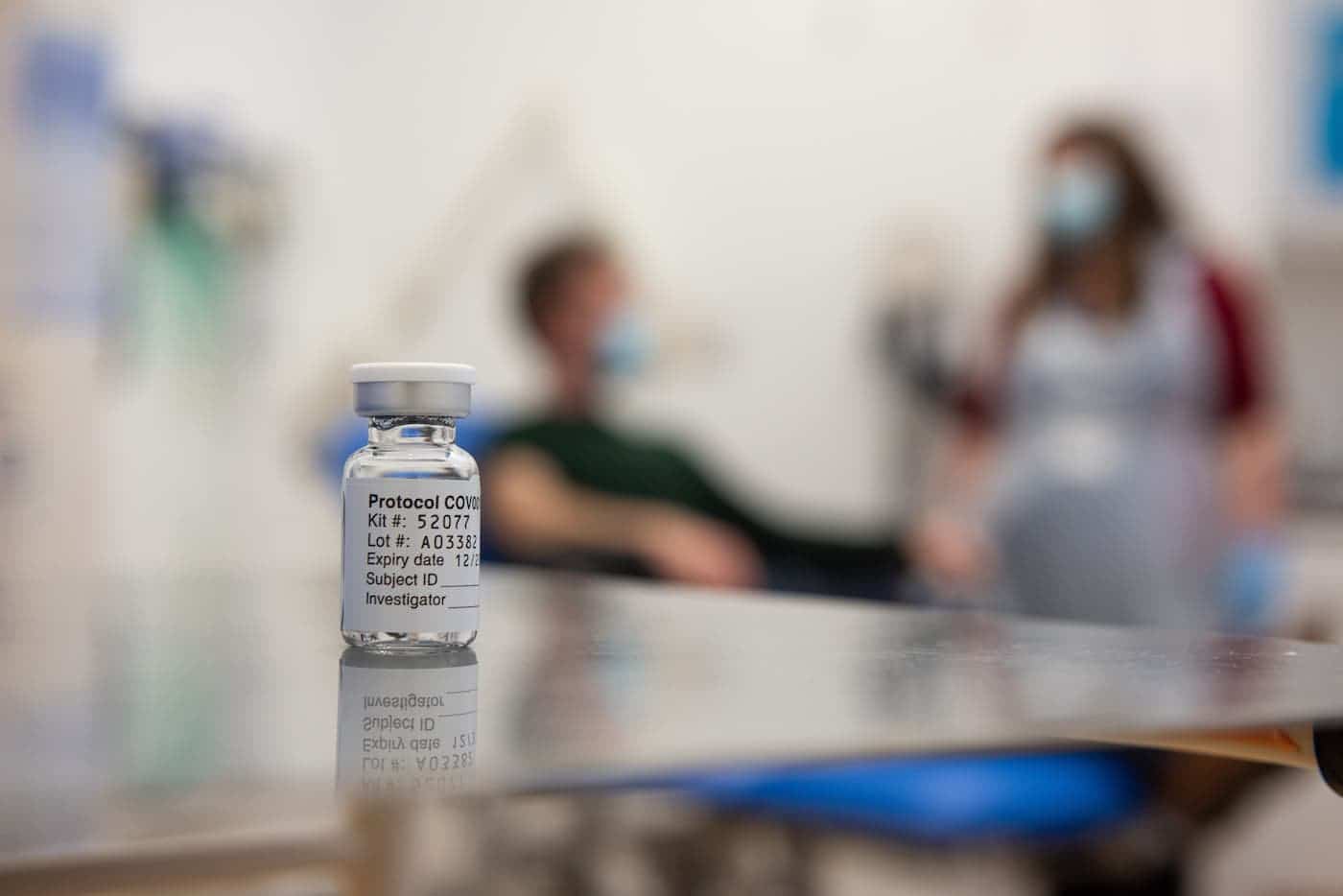
A Phase III study of the Oxford-AstraZeneca coronavirus vaccine conducted by AstraZeneca plc in the USA, Chile and Peru has shown that vaccine is safe and highly effective, adding to previous trial data from the United Kingdom, Brazil and South Africa, as well as real-world impact data from the United Kingdom.
In the trial, which recruited over 32,000 volunteers across all age groups, the participants received either two standard doses of the Oxford-AstraZeneca vaccine or a placebo vaccine, at a four-week interval.
These data show that the vaccine is 79% effective against symptomatic COVID-19, and 100% effective against severe, or critical symptomatic COVID-19.
These results also add to the extensive safety data collected both in previous trials and through real-world vaccine roll out schemes. The independent Data and Safety Monitoring Board (DSMB) reported no safety concerns among the participants receiving at least one dose of the vaccine.
The absolute efficacy is higher in this new study than observed in the Oxford-led studies, as efficacy is affected by the protocol case definition (higher for more severe cases) and the population in which the study is conducted.
Today’s findings are in line with findings from other major vaccine developers who studied efficacy in the US.
Andrew Pollard, Professor of Paediatric Infection and Immunity, and Lead Investigator of the Oxford University trial of the vaccine, said:
‘These results are great news as they show the remarkable efficacy of the vaccine in a new population and are consistent with the results from Oxford-led trials.
We can expect strong impact against COVID-19 across all ages and for people of all different backgrounds from widespread us of the vaccine.’
Sarah Gilbert, Professor of Vaccinology, and co-designer of the ChAdOx1 nCov-19 coronavirus vaccine, said:
‘These new results from the large phase III trials in the US, Chile and Peru provide further confirmation of the safety and effectiveness of ChAdOx1 nCoV-19.
In many different countries and across age groups, the vaccine is providing a high level of protection against COVID-19 and we hope this will lead to even more widespread use of the vaccine in the global attempts to bring the pandemic to an end.’
AstraZeneca will be submitting the data for analysis by the scientific community in peer-review literature, and to the regulators in the USA, the US Food and Drugs Administration (FDA) and for emergency approval for use.
For more about the Oxford vaccine project and team: www.ox.ac.uk/covid-vaccine